Lighthouse Pharma Direct Investment Page
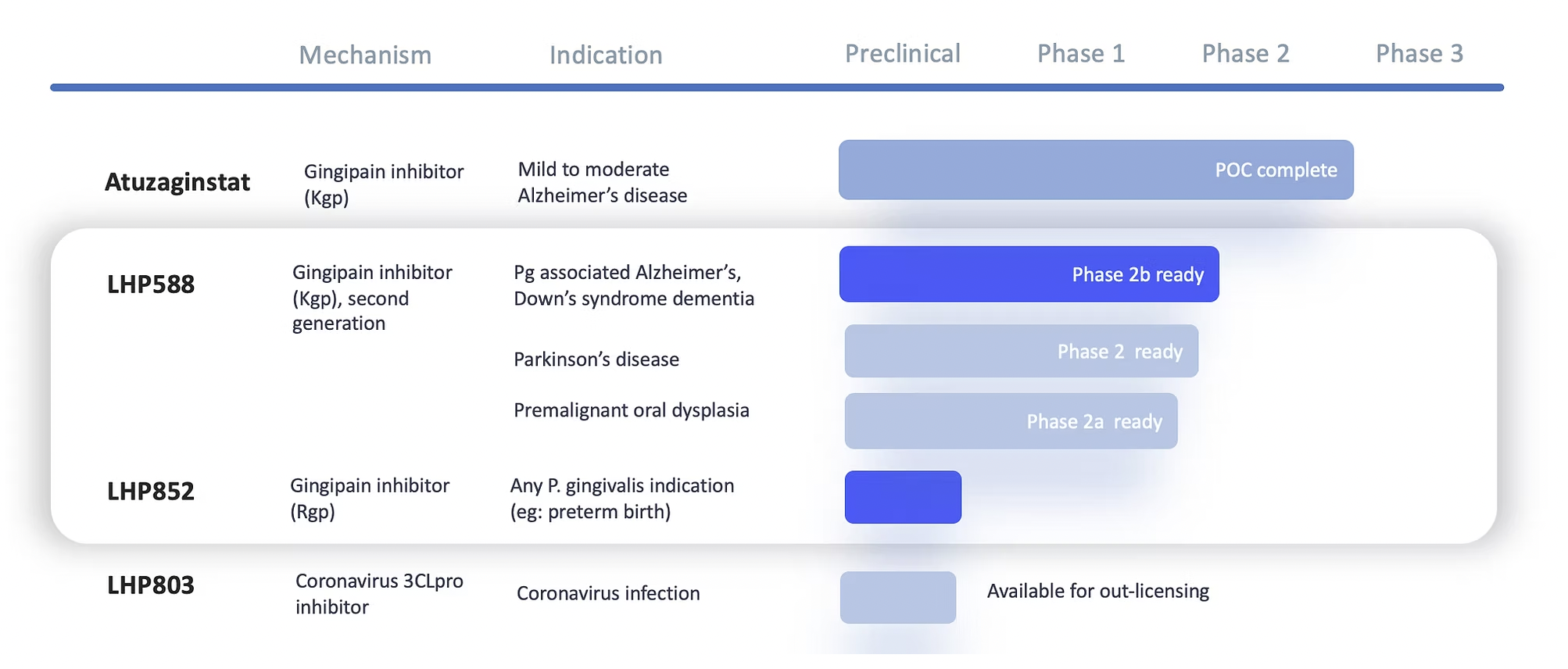
Lighthouse Pharma leverages translational insights and extensive clinical experience to better understand and treat dementia and other disorders. The team has a decade of data informing their strategy, including patient responder analyses, rigorous dose modeling, and clinical execution.
SPV Legal Name: Portfolia 2023 SPV 1 LLC (Lighthouse SPV) | EIN: 93-2652871
Portfolio Company | In the News
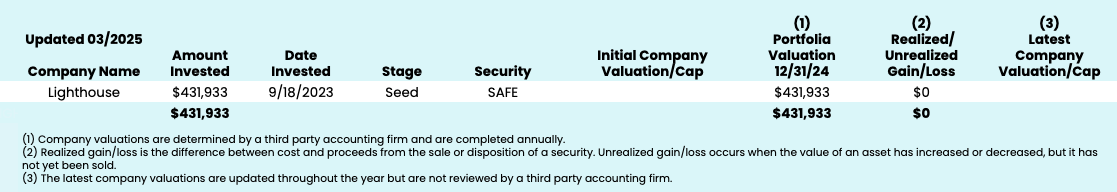
Fund Performance Overview
Quarterly Reports
The highlights of Q1 2025 updates as provided by the company. Please note all updates provided are confidential and not for distribution. To access the file, use password: Q125LighthouseSPV!
Moving forward, Portfolia will shift from quarterly to semi-annual company updates.
Videos
Company update and overview from CEO Casey Lynch | February 6, 2024